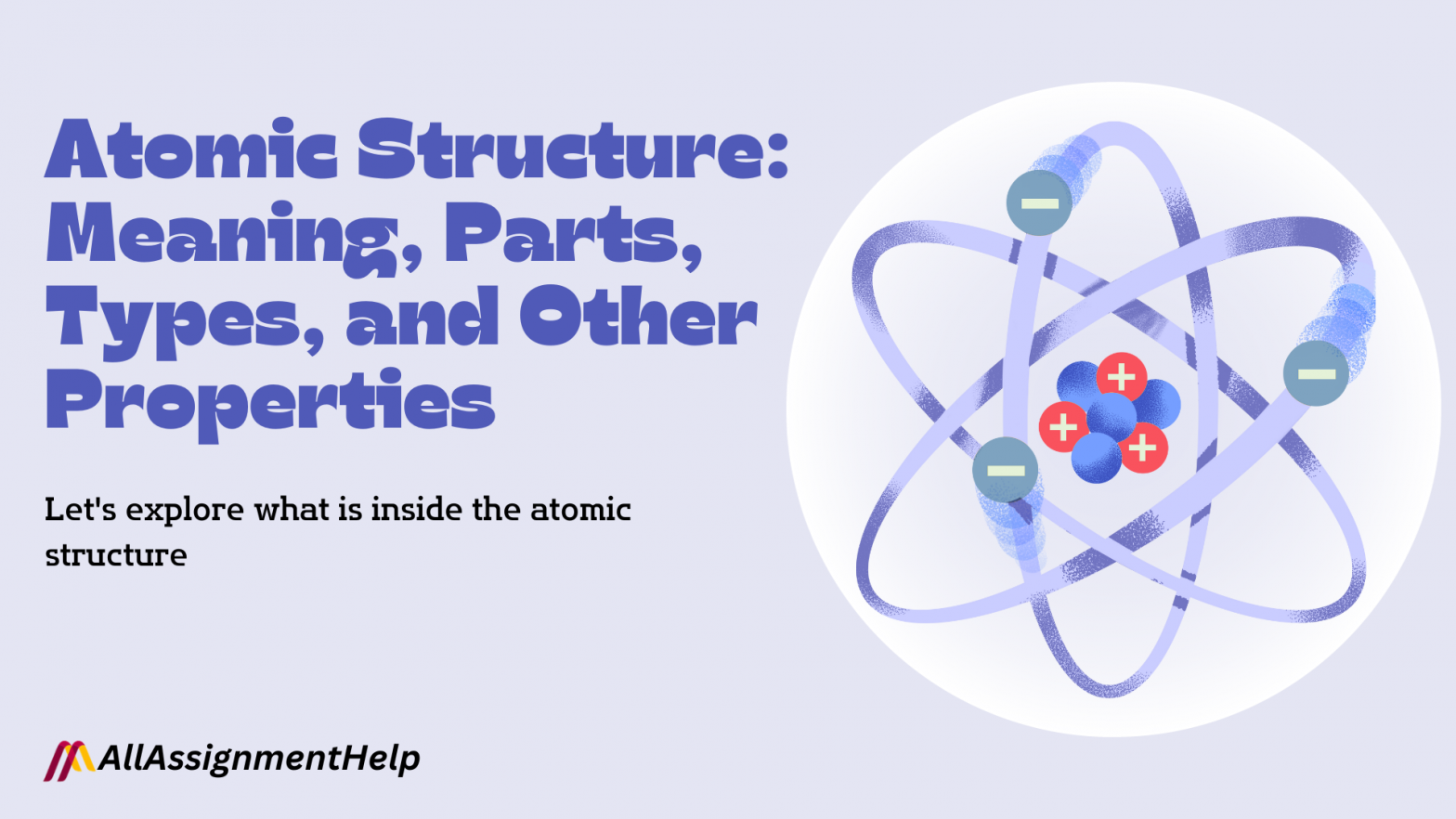
We have all studied the atom and its structure in our academics. The atomic structure of an element is all about the composition of its nucleus and the array of its electrons around the nucleus. This mainly consists of electrons, protons, and neutrons. It has been rightly said that the foundation of chemistry and the building block is an atom. Since all living and non-living material is made up of atoms, don’t you think it is important to know about its structure? An atom is a tiny portion of the element that participates in the chemical reaction. Atoms are made up of elements called electrons, protons, and neutrons. The organized arrangement of elements in an atom is called the atomic structure of an element. The diagram of an atomic structure consists of a nucleus in the center and positively charged protons and neutrally charged neutrons in between them.
In this blog post from the experts at AllAssignmentHelp.com, we have briefly discussed atomic structure. Moreover, we have also discussed protons, neutrons, and electrons.
Atomic Structure: What Is It?
- An atom consists of a nucleus and a series of orbitals.
- The atomic number in the atomic structure describes the ratio of protons present inside the nucleus.
- Democritus is the person who laid the foundation for the introduction of atomic structure to the world. He further said that every matter is composed of atoms.
- The nucleus is situated in the center of the atom, and orbitals surround it.
- Further, the atom of an element is made up of three different types of sub-atomic particles, namely electrons, protons, and neutrons.
- The nucleus of the atom consists of protons, which are positively charged, and neutral neutrons. Electrons, which are negatively charged elements of an atom, revolve around the nucleus in many separate paths, which are called orbitals.
- In simple words, it can be said that the atomic structure of an element consists of positively charged protons and neutral neutrons in the nucleus and negatively charged electrons surrounding the nucleus.
- The atomic structure of various elements varies from each other because of the diverse types of elements present in them.
- Most students think that every element consists of the same atomic structure, but this is not true. Different elements may have different structures. Protons and electrons are the reason for this difference. Because of the difference between protons and electrons, their number and structure may change.
- If you are interested in knowing more about atomic structure, you may seek online assignment help. By getting your assignments done by professional writers who have good subjective knowledge and understanding, you may get the benefit of submitting high-quality assignment solutions.
Parts of an Atomic Structure
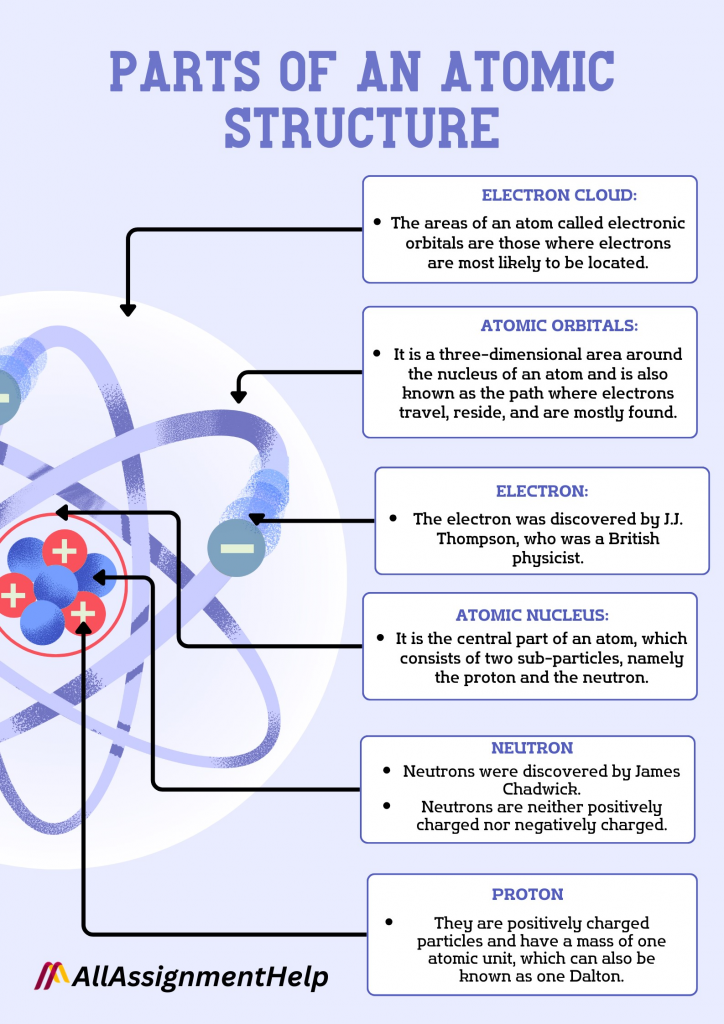
Do you know about the different parts of an atomic structure? It has been said that there are only three important components of structure. They are protons, electrons, and neutrons. Moreover, the nucleus is placed inside the atoms, and the outer orbit is known as the electron orbit. We have briefly explained the different parts of this structure right below. In case you found it difficult to understand these components, just type, Can I pay someone to take my online course on Google? That is it. With this search, you will be flooded with a lot of websites that are readily available to assist you with all of your online classes. Choose the one that seems appealing to you, and you may seek help from them.
Let us now see what the different parts of atomic structure are briefly in the below-given paragraphs.
Nucleus
- It is the central part of an atom, which consists of two sub-particles, namely the proton and the neutron.
- It is held together by a “strong force.” The elements of the nucleus are combined to form the overall atomic structure.
- The nucleus is known to have both positive protons and electrically neutral neutrons. It contains 0.01% of the volume of the atom and 99.9% of the mass of the atom.
- It is essential for the functioning of an atom’s structure and helps the orbits stay stable.
- Most of the time, it stays positive, as it is a combination of both positive and neutral charges (both positive and negative).
The Protons
- Rutherford is the person who first discovered the proton. The protons of an atom are found inside the nucleus of the atom, which is in the center.
- They are positively charged particles and have a mass of one atomic unit, which can also be known as one Dalton.
- It is the element having a positive charge, indicated by “P’. It has a fixed charge of 1.6*10^-19 Columbus, and the relative mass of this element is 1.6*10^-24 g.
- In the nucleus of every atom, one or more protons are present. This is one of the necessary elements of the nucleus to present an atomic structure. The number of these elements present in the nucleus of an atom is considered a significant property of each element.
- The total number of protons present in the atom is called the atomic number of that element and is represented by “z.”
- Every element has different numbers of protons, and this is why every element has a different atomic number.
- For example, hydrogen has only one proton, carbon has a total of six protons, and oxygen has eight protons.
- The presented number of protons in an element determines what kind of element it is. The numbers of these particles are also used to determine the chemical nature of the elements.
The Electron
- The electron was discovered by J.J. Thompson, who was a British physicist.
- An electron is indicated by “e” and is a negatively charged element of an atom.
- Electrons are found to be orbiting the nucleus.
- The fixed charge of this element is a negative charge of 1.6 *10^-19 Columbus, as well as a comparative mass of it that is small and calculated as 0 = 1/1836.
- Electrons spin around the nucleus of an atom in orbital paths. These are smaller than a neutron as well as a proton. These particles are more than 1800 times smaller compared to either a neutron or a proton.
- The nature of electrons is used by physicists to determine the properties of an atom, such as boiling point, stability, and conductivity. The electrons in an atom are arranged in a significant way.
- The electrons are found in the electron shells outside the nucleus. The electrons are negatively charged particles in an atom. The atomic structure of an element also takes into account the mass of the atom as well as the charge of the atom.
- The arrangement of electrons in an atom refers to the orbital definition of the location of these electrons in the atom. They are arranged depending on the potential energy in various orbits. The inner orbitals revolving around the nucleus are spherical, whereas the outer orbitals have more complicated configuration.
- The energy level is represented by 1, 2, 3, 4, and so on, and the orbits are K, L, M, N, and so on. They can change their orbits. The electrons are attracted to protons.
- The number of electrons in an atom is always equal to the number of protons present in that element. The number of electrons in an atom can change and determine the ion of the atom.
The Neutron
- Neutrons were discovered by James Chadwick.
- The neutrons are found in the nucleus of the atom. Its particles have an atomic mass of one unit, which is also known as one dalton.
- Neutrons are neither positively charged nor negatively charged. These are neutral. It is represented by the “n” and has no charge. This element of the atomic structure is neutral, having no charge.
- The relative mass of this element of an atom is the same as that of a proton, which is 1.6*10^-24. This element is available in the nucleus of an atom.
- The whole number of neutrons in an atom is called the neutron number. The mass of the neutron is a little larger compared to that of a proton.
- The chemical as well as the nuclear properties of an element are examined by both the number of protons and the number of neutrons present in the nucleus. This is a significant element necessary for the stability of the nucleus.
- Neutrons present in an atom can change its relative mass because the weights of the neutrons are equal to the weights of the proton and electron together.
- The total number of neutrons, together with the protons, is considered an atomic mass number. The number of neutrons available in the nucleus of an atom can be different from that of others, which differentiates the isotopes of an element from others.
Electron Orbit
- The areas of an atom called electronic orbitals are those where electrons are most likely to be located.
- It is a three-dimensional area around the nucleus of an atom and is also known as the path where electrons travel, reside, and are mostly found.
- There are a set number of orbitals in each subshell. As an illustration, consider the orbitals s = 1, p = 3, d = 5, and f = 7.
- It is stated that the greatest number of electrons that can exist in any orbital is two. No orbital can have three electrons or more above this.
Also Read: Tips and Tricks for Excelling in Online Physics Class
Other Important Properties of an Atomic Structure

In addition to the proton, electron, and neutron, there are also other basic properties of atomic structure. They are:
Atomic Mass
The mass of an atom is determined by the total mass of the neutrons and protons in the atom. This is because the neutrons and protons in the atom have a mass of one atomic unit, also known as one Dalton. Mathematically speaking, the mass of a neutron or a proton is around 1.67 x 10^-24 grams. On the other hand, the mass of an electron is ignored while calculating the mass of an atom. This is because the mass of an electron is around 1/8000th of one atomic mass unit. Its mass is very insignificant in terms of protons and neutrons, and it only weighs around 9.11×10^-28 grams. Hence, the greatest contribution while determining the mass of an atom is that of a neutron, while the least contribution while determining the total mass of an atom is that of an electron.
Atomic Charge
The atomic structure of an element also includes its atomic charge. Es have high contributions to the charge of an atom. This is because the electrons are positively charged, equal to the negative charge of the protons. The charge of the electrons is shown as -1, whereas the charge of the electrons is shown as +1. Neutrons have no charge, and consequently, they make no contributions to the charge of the atom. Studies hold that, in an atom that ought to be neutrally charged, the number of protons will be equal to the number of neutrons. Hence, the positive as well as the negative charges will cancel out each other.
Volume of Atoms
If the total size of protons, electrons, and neutrons were taken into account, it would be evident that almost the entire volume of the atom is empty. Despite that, solid objects are impenetrable. This can be attributed to the fact that the electrons present in the atom are negatively charged, and hence they cause repulsion with one another. This is what keeps the atoms from occupying the same place. The mass number of an atom, as well as the atomic number of an atom, also determines the atomic structure.
Atomic Number
The number of protons that are present determines the atomic number of an element. It is the number of protons, or atomic number, that distinguishes one element from another. However, the number of neutrons and electrons is a variable. The neutrons can vary in number, and consequently, they produce isotopes. The electrons of an element can also vary in number in the atoms, and they end up producing ions. An example of carbon can be taken, which has an atomic number of 6 because the number of protons present is 6.
Mass Number
The mass number of an atom is determined by dividing the total number of protons by the total number of neutrons. As mentioned earlier, the protons and neutrons of an atom have a similar mass, and due to the negligible mass that electrons possess, this cannot be taken into consideration. However, it is worth mentioning that the isotopes of an element will have the same atomic number, but they will have varying mass numbers.
For determining or calculating the mass of an atom, the arithmetic mean of the mass numbers of the isotopes of the element that occur naturally is calculated. To cite an example, the mass of an atom of the element chlorine is found to be 35.45 atomic mass units, and this is because chlorine has several naturally occurring isotopes, most of whose atomic masses are either 35 amu with a combination of 17 protons and 18 neutrons or 37 amu with a combination of 17 protons and 20 neutrons.
Types of Atomic Structure and Models
The term atom is derived from the Greek word “atom which refers to the smallest and most indivisible particle of an element. Protons, neutrons, and electrons are the three important parts of an atom. The atomic structure is something that briefly studies these three and helps us understand more about the functioning of atoms. Different scientists tried to explain the atoms and atomic structure in the 18th and 19th centuries with the help of atomic models. Whereas different atomic models were introduced, however, only a few of them became popular. We have listed the notable atomic structure models right below.
1- Dalton’s Atomic Structure Theory
- The English chemist John Dalton is the one who introduced this atomistic theory of structure to the world.
- He stated that every matter is made up of atoms, which are not divisible and not destroyable. Furthermore, he explained that each element will have a similar number of atoms, but different elements of atoms will have different masses and sizes of atoms.
- One of the important findings of his theory was that “atoms can neither be created nor destroyed but can be transformed from one form to another.”
- There were also a few demerits to Dalton’s theory. It failed to explain the existence of isotopes; the atomic structure was not properly discussed. He stated that atoms are indivisible; however, future scientists have found that this is not true and that atoms are divisible and can be divided into particles.
Also Read: Atomic Numbers -The Periodic Table of Elements
2- J.J Thomson’s Model of Atom
- J.J. Thomson (an English scientist) has also made some contributions to the discovery of atomism’s structure and models. The Thomson atomic model structure was introduced in the early 1990s, which significantly affected the Cathode Ray Experiment (which is a tube made of glass with two openings).
- He stated that atoms have a neutral charge. Why? He explained that it contains both a positively charged particle and an electron-negatively charged particle. He has also contributed to the discovery of the electron and has been awarded a Nobel Prize for the discovery of “electrons.”
- Like the other theory, these too have some drawbacks, like Thomson not explaining how positive charge holds on the electron inside the atom and not stating the reasons for the stability of an atom.
3- Rutherford’s Atomic Model
- Rutherford is a student of J.J. Thompson.
- Initially, it was found that an atomic structure consists of only three particles: protons, neutrons, and electrons. However, he explained about a subatomic particle called the “nucleus.”
- The Alpha Ray Scattering Experiment is an atomic model introduced by Rutherford. In his alpha-ray scattering experiment, he took a gold sheet and stated that only one in 1000 rays got reflected by an angle of 180°after hitting the foil.
- His observations also stated that the gold ray is reflected because of the repulsion of its positive and that some α- particles passed through the gold foil without deflecting.
- In terms of atomic structure, he found that the size of nuclei is significantly smaller in the atom, electrons revolve around the nucleus and the path on which they travel is called the “orbit.”.
We have discussed the three major atomic structure models introduced by different scientists. If you are a chemistry student, you must be well aware of this atom structure. If you don’t know this and need any kind of expert assistance, visit assignment help online or an instant assignment help service and place your order. The professionals in this service are known to have different Ph.D. degree holders from renowned universities. They may assist you with any of your complex chemistry assignments on any topic, leading to good grades.
Struggling with Different Chemistry Concepts? Get Help From Us
Are you facing a lot of problems learning chemistry concepts? Do topics like general organic chemistry, ionic and chemical equilibrium, thermodynamics, and atomic structure seem impossible for you to learn? Well, no worries; let the professionally talented writers at All Assignment Help handle your work. The students who reach out to us with requests like, Please, do my online course? or Can I pay you to take my online physics class? have found the right assistance at an affordable price. Our experts have expertise in every area, like normalized and orthogonal wave functions, postulates of quantum mechanics, oxidation, and reduction. So, what are you waiting for? Reach out to us now, and we will guide you in every way.
Our service is not limited to online class assistance only. We have expertise in providing exam help to students worldwide. To avail yourself of our service, you need to ask, Can you take my online test for me? Do not forget to mention which subject and type of exam you need us to take, so that we can assist you accordingly.
Frequently Asked Questions
| Question 1: What are electrons and protons? Answer 1: Electrons are negatively charged particles, though protons are positively charged particles. The positive charge is equivalent to the proton, and the negative charge is equivalent to the electron. |
| Question 2: What is atomic structure? Answer 2: An atomic structure is a combination of protons, neutrons, and electrons. It further consists of a nucleus in the center spot. |
| Question 3: What are isotopes? Answer 3: Isotopes are defined as the atoms of the same element, but they vary in the number of neutrons. However, they have the same number of protons. Even though the isotopes have a different number of neutrons, they do not have significant differences in their physical properties. |