Spectrum is a term derived from the word spectra. Are you confused about the meaning of this term and where it is used? It is a word that is mostly used in the field of optics. Furthermore, it refers to the wide range of wavelengths of different frequencies of radiation. A spectrum is classified into two types: emission spectra and absorption spectra. The radiation emitted by electrons in excited molecules or atoms is known as the emission spectrum. The absorption spectrum of a material can be defined as the fraction of the incident radiation that is absorbed by that material over a wide range of frequencies. The molecular and atomic composition of a material is used to determine the absorption spectrum. Fundamental radiation is generally observed at those frequencies that get mixed with the energy difference that takes place between two mechanical states of the molecules.
If you are interested in learning more about this concept, then this write-up is for you. This blog from AllAssignmentHelp.com will help you understand the absorption spectrum in a detailed manner. So let’s get started and explore more.
What Is the Absorption Spectrum?
The absorption spectrum of a material can be defined as the fraction of the incident radiation that is absorbed by that material over a wide range of frequencies. The absorption takes place because of the transition between these two states, which is known as the absorption line. The spectrum is composed of several absorption lines. The frequencies at which such absorption lines develop, along with their relative intensities, generally depend on the molecular structure and electronic structure of the sample. The frequencies also depend on molecular interactions. In the sample, the crystal structure is found in solids and depends on different environmental factors like pressure, temperature, electromagnetic fields, etc.
The absorption spectrum takes place when the light passes through a dilute and cold gas, and characteristic frequencies get absorbed by the atoms present in the gas. The re-emitted light cannot be emitted in a similar direction, which is followed by absorbed photons. Because of this, dark lines in the spectrum are created in the absence of light. The absorption spectrum is the dark line. The absorption spectrum is defined as an electromagnetic spectrum in which the radiation intensity at some specific wavelengths decreases. An absorbing substance manifests as bands or dark lines. Medically, the absorption spectrum is also defined as an electromagnetic spectrum in which radiation intensity at specific ranges of wavelength is manifested as dark lines.
The above was a short introduction to the absorption spectrum. This is an important concept in the optics degree, where you will learn more about absorption lines, how they work, and so on. If you think that learning about spectrum features is difficult, you should seek instant assignment help from professional writers. With their assistance, you may submit a well-written assignment and improve your grades and knowledge on these topics.
Absorption Line: Let’s Learn More About It
The absorption lines possess a definite shape and width, which are fundamentally determined by the density of states for the spectral density of the system. Below, we have discussed more about absorption lines in detail.
- Absorption lines are generally classified by the feature of a quantum mechanical change taking place in an atom or molecule.
- Vibrational lines in correspondence to vibrational state changes in the molecule are found in the infrared region.
- The electronic lines are composed of several changes taking place in the electronic state of a molecule or atom, which are found in the ultraviolet and visible regions.
- It can be noticed that there are various dark lines in the sun’s spectrum. These lines are developed by the atmosphere of the sun, which absorbs light at different wavelengths, resulting in different light intensities at each wavelength appearing dark. The molecules and atoms present in a gas absorb certain light wavelengths.
- Dark lines are the areas where the light gets absorbed by different elements present in the sun’s outer layers. The lowest energy is represented by red light, and the highest energy is represented by blue light.
- The black gaps or lines in the spectrum of the sun are termed absorption lines. The gas present in the sun’s outer layers develops the absorption lines by absorbing the light. There are different elements in the sun, such as helium, hydrogen, carbon, and other smaller quantities of heavy elements.
- Absorption lines of any gas-phase molecule can get shifted typically when the molecule is present in the solid or liquid phase and involved in strongly interacting with neighboring molecules. The shape and width of the absorption lines are generally determined by the observation instrument. The physical environment, radiation, and material absorption also determine the shape and width of absorption lines.
Relation Between Transmission and Absorption Spectra
Transmission and absorption spectra are interconnected and represent similar information. The transmission spectrum can be calculated from the absorption spectrum only. The absorption spectrum can also be calculated from transmission spectra. A mathematical transformation is used in calculating either the absorption spectrum or the transmission spectrum. It has been observed that a transmission spectrum has maximum intensities where the wavelengths of the absorption spectrum are quite weak because the transmission of more light through the sample takes place. Similarly, an absorption spectrum is found to have maximum intensities at its wavelengths, where the absorption rate is quite strong.
Also Read: Biology Assignment: A Full Package Guide
Absorption Spectrum: Relationship Between Reflection and Scattering Spectra
The absorption spectrum is also related to reflection and scattering spectra. The scattering and reflection spectra are influenced by the absorption spectrum and index of refraction. The extinction coefficient quantifies the absorption spectrum and index coefficients along with the extinction coefficients, which are related quantitatively through the Kramers-Kroening relation. Therefore, it can be said that reflection or scattering spectrum standardization of the absorption spectrum can give rise to the absorption spectrum.
Reflection or scattering spectrum assumptions or models need to be simplified so that they can lead to an approximation of the derivation of absorption spectra. In the domain of chemical analysis, absorption spectroscopy is used. The specificity enables the compounds to be distinguished from each other in a mixture, which makes absorption spectroscopy highly useful in different applications. For example, the presence of any pollutant in the air can be identified by the use of infrared gas analyzers.
These analyses are also used to distinguish the air pollutant from oxygen, water, nitrogen, and other constituents. The specificity is also helpful in allowing several unknown samples to be correctly identified. It has been found that the qualitative information of any sample can also be determined even if the information is not present in a library. For example, infrared spectra have several characteristic absorption bands that help indicate the presence of carbon-oxygen bonds or carbon-hydrogen bonds.
The absorption spectrum can also be related to the quantity of material present with the use of Beer-Lambert law. This relationship is established quantitatively. In determining the typical compound concentration, one needs knowledge of the absorption coefficient of the compound. The absorption coefficient can be known from several reference sources and can be measured by accessing a caliber standard spectrum with an available target concentration.
How Are the Absorption Spectrum and Emission Spectrum Related to Each Other?
The absorption spectrum is also related to any emission spectrum. Now, it is important to understand the concept of the emission spectrum. The process by which a substance can release energy is known as the emission process. The energy that is released from a substance through any emission process can be found in electromagnetic radiation. Emission can take place at any frequency of absorption, which makes the absorption lines determine the emission spectrum. But it is to be remembered that the emission spectrum will always have a different intensity pattern when it is distinguished from that of the absorption spectrum. Hence, it can be said that the absorption spectrum and emission spectrum can never be equivalent. The emission spectrum can be used to calculate the absorption spectrum with the application of effective theoretical models and other relevant information from which the quantum mechanical states of a substance can be understood.
- The absorption spectrum is the opposite of the emission spectrum; however, sometimes emission can occur at any frequency at which absorption can occur.
- The abortion spectrum and emission spectrum are not equivalent and follow different intensity patterns.
- Einstein’s coefficient is the main term used in calculating absorption spectra.
Five Advantages of the Absorption Spectrum
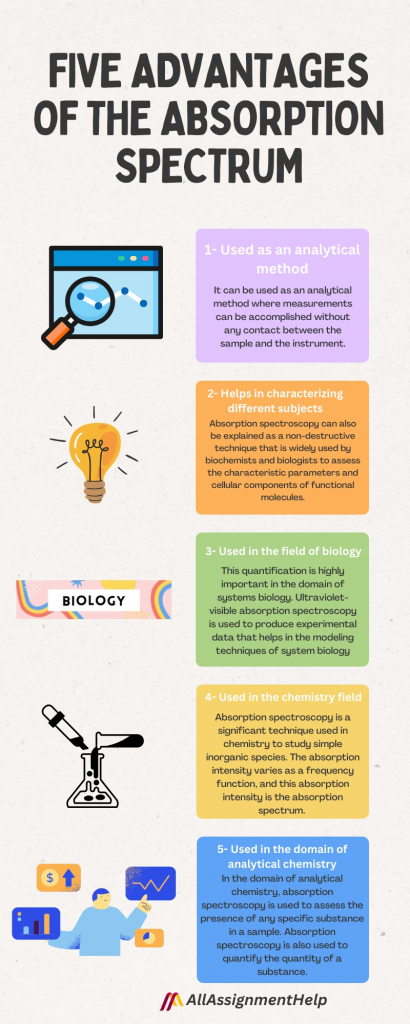
Absorption spectroscopy is a tool that is used to identify particular substances in a given sample. It is a technique that measures the absorption of electromagnetic radiation. There are also several other advantages to absorption spectroscopy. Some of the advantages are listed as follows:
1- Used as an analytical method
It can be used as an analytical method where measurements can be accomplished without any contact between the sample and the instrument. Radiation that travels between an instrument and a sample contains some important spectral information, and measurement is done remotely. Remote spectral sensing is quite significant in different situations. For example, hazardous and toxic environments can be measured without risking any instrument or operator.
2- Helps in characterizing different subjects
Absorption spectroscopy is one of the methods with the help of which a substance can be characterized by the support of wavelengths at which the spectrum of color gets absorbed during the passage of light through a substance solution. It is one of the most fundamentally used methods for assessing the chromosphere concentrations in solutions. Absorption spectroscopy can also be explained as a non-destructive technique that is widely used by biochemists and biologists to assess the characteristic parameters and cellular components of functional molecules.
3- Used in the field of biology
This quantification is highly important in the domain of systems biology. In developing a quantitative metabolic pathway depiction, various variables and parameters are needed that are to be assessed experimentally. Ultraviolet-visible absorption spectroscopy is used to produce experimental data that helps in the modeling techniques of system biology. These techniques use kinetic parameters and concentrations of enzymes for signaling metabolic pathways, fluxes, and intercellular metabolic concentrations. Absorption spectroscopy also describes the use of the technique in quantifying biomolecules and investigating biomolecular interactions.
Are you pursuing courses in the field of biology? Do you find dissertations to be the most complicated part of your courses? Seek biology dissertation help from online writers and relieve your academic stress. The online helpers will help you submit a well-crafted biology dissertation and score high marks.
4- Used in the chemistry field
Absorption spectroscopy is a significant technique used in chemistry to study simple inorganic species. It refers to spectroscopic techniques that are used in measuring radiation absorption as a function of wavelength or frequency when the interaction between absorption radiation and the sample takes place. Photons are absorbed by the samples from the field of radiation. The absorption intensity varies as a frequency function, and this absorption intensity is the absorption spectrum. Absorption spectroscopy is fundamentally performed across an absorption spectrum or electromagnetic spectrum.
5- Used in the domain of analytical chemistry
In the domain of analytical chemistry, absorption spectroscopy is used to assess the presence of any specific substance in a sample. Absorption spectroscopy is also used to quantify the quantity of a substance. In the domain of analytical applications, ultraviolet-visible and infrared spectroscopy are commonly observed. The study of atomic physics, remote sensing, molecular physics, and astronomical spectroscopy, the use of absorption spectroscopy is widely observed. Students studying analytical chemistry often struggle to understand absorption spectroscopy. If you are also one who thinks that dealing with your academic pressure is difficult, then you should hire professional experts from online assignment help services.
There are various experimental approaches that are used to measure the absorption spectrum. The most commonly used arrangement is to guide the regenerated radiation beam at the sample in detecting the radiation intensity passing through it. The transmitted energy can be applied to calculate the absorption. The sample arrangement, source, and detection technique are also very widely used, depending on the objective of the experiment and the frequency range.
Also Read: Biology homework help – For best Grades
About Us
Is managing your online classes and maintaining good grades an impossible task for you? Are you surrounded by thoughts like, I need someone to take my class for me? Nowadays, academic pressure has become unmanageable. Both school and university students are facing a lot of issues in earning good marks on their assignments, exams, discussions, quizzes, and projects. These are the reasons that have enabled them to hire online writers for their rescue. We at All Assignment Help have some highly qualified classtakers to help you with all of your academic concerns. Our professionals are experts in different fields and can assist you at any time and earn you high grades.
If you want us to take your exams, just ask, Can you take my online exam? and we will respond to you in no time. Our specialists can complete all of your midterm and final exams within a day. So what are you waiting for? Reach out to us, improve your academic scores, and reduce your academic burden.
Frequently Asked Questions
| Question 1: How are absorption spectra observed? Answer 1: The absorption spectra can be observed from spatial regions in the presence of a cooler gas line between a hotter source and the Earth. The absorption spectra can also be observed on planets with atmospheres, stars, and galaxies. |
| Question 2: What is a spectrometer? Answer 2: The spectrometer is a device that separates light by color and energy. A spectrometer is used to analyze the light of the sun. In separating light by color and energy, the image of the spectrum of the sun gets created. |
| Question 3: What are the different types of absorption spectrum? Answer 3: Line absorption spectrum, band absorption spectrum, and continuous absorption spectrum are the three major types in this spectrum. |
| Question 4: What approaches are there in spectroscopy? Answer 4: There are different approaches to spectroscopy. A few of them are astronomical spectroscopy, photoacoustic spectroscopy, laser absorption spectrometry, reflection-absorption infrared spectroscopy, and tunable diode laser absorption spectroscopy (TDLAS). |
